Introduction:
Discover the fascinating world of Nuclear Magnetic Resonance (NMR) spectroscopy through our comprehensive guide. This innovative scientific technique has revolutionized research and analysis, and yields valuable insights into the molecular world. Our article delves into the intricacies of NMR spectroscopy, exploring its principles, applications, and ways it enhances our understanding of science. Suitable for students, researchers, and the curious alike, our guide provides the knowledge to unlock the secrets of this captivating science.
- Understanding the Basics of NMR Spectroscopy:
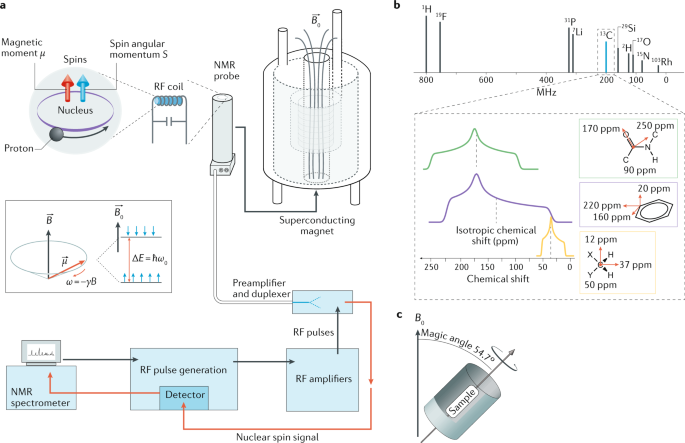
Utilizing the magnetic properties of atomic nuclei, Nuclear Magnetic Resonance spectroscopy (NMR) is a highly effective analytical technique used to investigate atomic nuclei within a magnetic field. NMR spectroscopy offers valuable insights into the makeup, configuration, and motion of molecules. The fundamental concept behind NMR spectroscopy relies on the resonance phenomenon, whereby specific atomic nuclei take in and then discharge electromagnetic radiation at precise frequencies.
- How Does NMR Spectroscopy Work?
NMR spectroscopy is based on the interplay between atomic nuclei and magnetic fields. When a sample is exposed to a strong magnetic field and radiofrequency waves, the nuclei align themselves with the field. By manipulating the nuclei with further radiofrequency impulses, they can switch between different energy states. By emitting and detecting radiofrequency signals, essential molecular properties, including chemical shifts, coupling constants, and relaxation times can be established.
- Applications of NMR Spectroscopy:
NMR spectroscopy is a versatile tool utilized in numerous scientific fields, such as chemistry, biochemistry, materials science, and pharmaceutical research. Take a closer look at the vital roles NMR spectroscopy plays in the following key areas:
3.1. Structural Determination:
Explore the intricate structures of organic and inorganic compounds with the indispensable aid of NMR spectroscopy. By analyzing chemical shifts, spin-spin couplings, and other spectral features, researchers can unravel the atom connectivities, unlock molecular conformations, and delve into the dynamics of molecules. Leverage this powerful tool to gain invaluable insights into the complex building blocks of our world.
3.2. Drug Discovery and Development:
NMR spectroscopy is an essential tool in drug discovery and development in the pharmaceutical industry. Researchers utilize it to examine potential drug molecules and their interactions with target proteins to enhance drug candidates, evaluate binding affinities, and uncover mechanisms of action.
3.3. Metabolomics and Biomolecular Analysis:
Metabolomics is the study of small molecule metabolites in biological systems. To fully understand disease mechanisms, biomarker discovery, and personalized medicine, it’s essential to identify and quantify metabolites in complex biological samples. Fortunately, NMR spectroscopy is a reliable technique used to achieve these goals.
3.4. Material Science and Quality Control:
Discover the secrets hidden within materials with NMR spectroscopy. This technique unlocks invaluable insights into physical and chemical properties, from polymers to catalysts. Explore composition, structure, and dynamics to optimize synthesis processes, assess material quality, and engineer superior materials with enhanced properties. Our team of experts is ready to help you unlock the full potential of your materials.
- Advanced NMR Techniques:
NMR spectroscopy, beyond its fundamental principles, offers a diverse range of advanced techniques that can take your research to the next level. Noteworthy techniques include:
4.1. Multidimensional NMR:
Discover complex molecules like never before through the use of Multidimensional NMR techniques. Obtain highly resolved spectra with the ability to analyze multiple spectra along different dimensions, making spectral interpretation easier and enabling in-depth determinations of molecular structures.
4.2. Solid-State NMR:
Solid-state NMR is a powerful technique that expands beyond the limitations of conventional NMR spectroscopy, which focuses on liquid samples. It allows for the exploration of a wide range of materials with varied structures, including catalysts, polymers, and crystals. By providing valuable insights into local environments, molecular dynamics, and phase transitions, this technique has contributed significantly to the advancements in materials science and catalysis research.
4.3. Protein NMR:
Protein NMR spectroscopy is essential for structural biology and drug discovery. Through the analysis of protein NMR spectra, researchers can identify the 3D structure of proteins, explore protein-ligand interactions, and investigate the dynamics of biomolecular systems. This information is crucial for comprehending protein folding, enzymatic mechanisms, and designing precise therapeutics.
- Overcoming Challenges in NMR Spectroscopy:
Despite its great analytical potential, using NMR spectroscopy poses certain challenges. Amongst these, there are sensitivity constraints, spectral overlap, and problems with sample preparation. Fortunately, technology advancements such as higher field strengths, cryogenic probes, and refined pulse sequences have overcome these obstacles, allowing complex samples to be analyzed with a higher degree of precision and detail.
- Future Perspectives and Innovations:
With technology rapidly evolving, NMR spectroscopy stands poised for further innovation and expanded applications. Stay up-to-date with emerging trends and developments in this field, including:
6.1. Hyperpolarization Techniques:
Discover the unseen with hyperpolarization techniques in NMR spectroscopy. Increase sensitivity by selectively boosting the population of targeted nuclear spin states. Employ dynamic nuclear polarization (DNP) and parahydrogen-induced polarization (PHIP) to amplify NMR signals. Unveil the secrets of elusive molecules and processes with the power of hyperpolarization.
6.2. In-cell NMR:
Explore the inner workings of living cells with the power of in-cell NMR spectroscopy. With isotopically labeled molecules and NMR techniques, researchers can now gain valuable insights into cellular metabolism, protein-protein interactions, and intracellular signaling pathways. Discover the true potential of in-cell NMR in unlocking the mysteries of cellular biology in real-time.
6.3. Hybrid Methods:
By incorporating NMR spectroscopy alongside other analytical methods like mass spectrometry, electron microscopy, and X-ray crystallography, scientists can gain a comprehensive comprehension of molecular structures and interactions. With these hybrid techniques, researchers can merge the advantages of diverse methodologies, leading to groundbreaking developments in numerous scientific fields.
Conclusion:
Nuclear Magnetic Resonance (NMR) spectroscopy has revolutionized our understanding of the molecular world. Its power as an analytical technique has made it relevant across various scientific fields, from chemistry and biochemistry to materials science and pharmaceutical research. By providing valuable insights into molecular structures, dynamics, and interactions, it continues to drive innovation and discoveries. Discover how this analytical technique can transform the scientific field to greater heights.
GO BACK TO THE MAIN PAGE



